Australia's approach to trade rules restraining vaccine production
- Written by Deborah Gleeson, Associate professor, La Trobe University
Papua New Guinea’s COVID-19 outbreak is a portent of the “catastrophic moral failure[1]” the head of the World Health Organization warned of in January due to poor countries being pushed to the back of the vaccine queue.
Australia has gifted 8,000 doses to PNG, and vowed to help the nation of almost 9 million secure 1 million more[2]. Earlier this month Australia agreed to work with the US, India and Japan to provide 1 billion vaccines[3] to poorer countries in the Asia-Pacific. It is also supporting COVAX[4], the global program aiming to buy and distribute 2 billion COVID-19 vaccine doses to developing nations by the end of 2021.
But all this could be negated through Australia’s potential spoiling role (with a handful of other countries) against a proposal supported by 118 countries to ramp up vaccine production by relaxing the trade rules governing intellectual property.
Inequities in access to COVID-19 vaccines
The inequities in access to vaccines are stark. By November, wealthy nations accounting for just 14% of the global population[5] had locked in premarket agreements to buy 51% of the first 7.48 billion doses of candidate COVID-19 vaccines.
That number should be enough to vaccinate almost half the global population – 3.76 billion of 7.8 billion people. But Canada, Australia, Britain, Japan, the European Union and the US have all bought up more than their fair share. Canada has reserved about 4.5 courses per person; Australia and the UK close to 2.5.
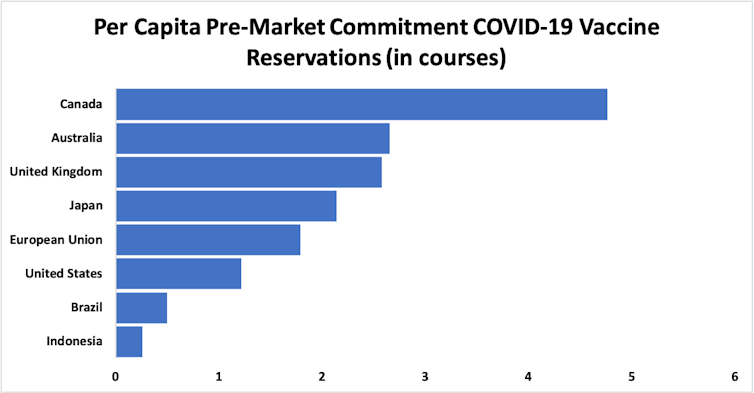 Anthony D. So/British Medical Journal[6]
This means most middle-income countries won’t achieve widespread vaccination coverage until late 2022, according to The Economist’s Intelligence Unit[7]. For poorer economies, including some of Australia’s closest neighbours, it won’t be “before 2023, if at all”.
Anthony D. So/British Medical Journal[6]
This means most middle-income countries won’t achieve widespread vaccination coverage until late 2022, according to The Economist’s Intelligence Unit[7]. For poorer economies, including some of Australia’s closest neighbours, it won’t be “before 2023, if at all”.
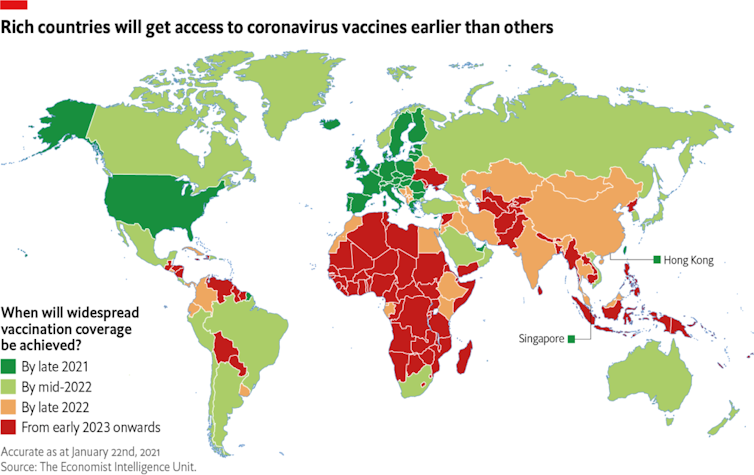 The Economist Intelligence Unit[8]
Suspending intellectual property rules
Addressing vaccine inequities, the director-general of the World Health Organization, Tedros Adhanom Ghebreyesus has said, requires pulling “out all the stops[9]”.
One of those stops is the international agreement protecting intellectual property rights – the Agreement on Trade-Related Aspects of Intellectual Property Rights, commonly called the TRIPS agreement[10]. All members of the World Trade Organization are bound by the agreement.
In October 2020, South Africa and India proposed waiving[11] certain provisions of TRIPS that give the owners of the intellectual property exclusive rights over the manufacture and sale of COVID vaccines. The waiver would only apply to COVID-19 medical products and only for the duration of the pandemic. This would allow others to make COVID-19 vaccines without the vaccine developers’ permission.
The Economist Intelligence Unit[8]
Suspending intellectual property rules
Addressing vaccine inequities, the director-general of the World Health Organization, Tedros Adhanom Ghebreyesus has said, requires pulling “out all the stops[9]”.
One of those stops is the international agreement protecting intellectual property rights – the Agreement on Trade-Related Aspects of Intellectual Property Rights, commonly called the TRIPS agreement[10]. All members of the World Trade Organization are bound by the agreement.
In October 2020, South Africa and India proposed waiving[11] certain provisions of TRIPS that give the owners of the intellectual property exclusive rights over the manufacture and sale of COVID vaccines. The waiver would only apply to COVID-19 medical products and only for the duration of the pandemic. This would allow others to make COVID-19 vaccines without the vaccine developers’ permission.
 Medecins Sans Frontieres members deploy a banner in front of the headquarters of the World Trade Organization in Geneva on March 4 2021.
Martial Trezzini/EPA
The TRIPS waiver is supported by 118[12] of the WTO’s 164 members – more than two-thirds of its membership.
The counter-proposal supported by Australia
Australia, however, has joined with Canada, Chile, Colombia, New Zealand, Norway and Turkey in supporting a counter-proposal[13] that argues provisions already in the TRIPS agreement are good enough.
TRIPS does allow for compulsory licensing, enabling the use of a patent without the consent of the owner, in a public health emergency. But the compulsory licensing process is time-consuming and only applies to patents, not the other types of intellectual property important for making vaccines.
Medecins Sans Frontieres members deploy a banner in front of the headquarters of the World Trade Organization in Geneva on March 4 2021.
Martial Trezzini/EPA
The TRIPS waiver is supported by 118[12] of the WTO’s 164 members – more than two-thirds of its membership.
The counter-proposal supported by Australia
Australia, however, has joined with Canada, Chile, Colombia, New Zealand, Norway and Turkey in supporting a counter-proposal[13] that argues provisions already in the TRIPS agreement are good enough.
TRIPS does allow for compulsory licensing, enabling the use of a patent without the consent of the owner, in a public health emergency. But the compulsory licensing process is time-consuming and only applies to patents, not the other types of intellectual property important for making vaccines.
 A vaccination centre in Mumbai, India, on March 10 2021.
Rajanish Kakade/AP
The counter-proposal put its faith in encouraging more talk between vaccine developers and manufacturers, enabling them to make voluntary licensing agreements and identify ways to increase production.
But there’s little reason to think this would achieve more than what is already happening. For example, AstraZeneca has licensed the Serum Institute of India[14] to make its vaccine both for India and other countries.
Read more:
Yes, export bans on vaccines are a problem, but why is the supply of vaccines so limited in the first place?[15]
Voluntary licences are problematic because they are ad hoc and confidential. The lack of transparency has seen pricing discrepancies[16] such as Bangladesh and South Africa reportedly paying more for their AstraZeneca doses than European nations.
Voluntary licences can also place tight restrictions on where the resulting products can be sold. For example, when US pharmaceutical company Gilead Sciences negotiated licences with makers in India, Pakistan and Egypt to produce a cheaper version of Remdesivir (a candidate for COVID-19 treatment in the early stages of the pandemic) the licence conditions excluded many middle-income countries[17] from buying the cheaper version.
Australia’s support for this counter-proposal is therefore a distraction at best. It can’t bring about the fundamental change the TRIPS waiver could generate. Nor is it likely to lead to the world being vaccinated sooner.
A vaccination centre in Mumbai, India, on March 10 2021.
Rajanish Kakade/AP
The counter-proposal put its faith in encouraging more talk between vaccine developers and manufacturers, enabling them to make voluntary licensing agreements and identify ways to increase production.
But there’s little reason to think this would achieve more than what is already happening. For example, AstraZeneca has licensed the Serum Institute of India[14] to make its vaccine both for India and other countries.
Read more:
Yes, export bans on vaccines are a problem, but why is the supply of vaccines so limited in the first place?[15]
Voluntary licences are problematic because they are ad hoc and confidential. The lack of transparency has seen pricing discrepancies[16] such as Bangladesh and South Africa reportedly paying more for their AstraZeneca doses than European nations.
Voluntary licences can also place tight restrictions on where the resulting products can be sold. For example, when US pharmaceutical company Gilead Sciences negotiated licences with makers in India, Pakistan and Egypt to produce a cheaper version of Remdesivir (a candidate for COVID-19 treatment in the early stages of the pandemic) the licence conditions excluded many middle-income countries[17] from buying the cheaper version.
Australia’s support for this counter-proposal is therefore a distraction at best. It can’t bring about the fundamental change the TRIPS waiver could generate. Nor is it likely to lead to the world being vaccinated sooner.
References
- ^ catastrophic moral failure (www.bbc.com)
- ^ 1 million more (www.abc.net.au)
- ^ to provide 1 billion vaccines (www.theguardian.com)
- ^ COVAX (www.who.int)
- ^ 14% of the global population (www.bmj.com)
- ^ Anthony D. So/British Medical Journal (www.ignitetheidea.org)
- ^ The Economist’s Intelligence Unit (www.eiu.com)
- ^ The Economist Intelligence Unit (www.eiu.com)
- ^ out all the stops (www.theguardian.com)
- ^ TRIPS agreement (www.wto.org)
- ^ proposed waiving (docs.wto.org)
- ^ 118 (www.twn.my)
- ^ counter-proposal (docs.wto.org)
- ^ Serum Institute of India (economictimes.indiatimes.com)
- ^ Yes, export bans on vaccines are a problem, but why is the supply of vaccines so limited in the first place? (theconversation.com)
- ^ pricing discrepancies (www.politico.eu)
- ^ excluded many middle-income countries (www.citizen.org)
Authors: Deborah Gleeson, Associate professor, La Trobe University













